Learning Objective
Ionic radius Ions are particles in the same way as atoms only they carry an electrical charge. This is due to the number of electrons in the outer shells. If a particle has one fewer electrons then it carries a positive charge (there is an excess of protons in the nucleus). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Search for more papers by this author.
- Identify the general trends of the ionic radius size for the periodic table.
Ionic Radii Definition
Key Points
- The ionic radius is the distance between the nucleus and the electron in the outermost shell of an ion.
- When an atom loses an electron to form a cation, the lost electron no longer contributes to shielding the other electrons from the charge of the nucleus; consequently, the other electrons are more strongly attracted to the nucleus, and the radius of the atom gets smaller.
- When an electron is added to an atom, forming an anion, the added electron repels other electrons, resulting in an increase in the size of the atom.
- The trend observed in size of ionic radii is due to shielding of the outermost electrons by the inner-shell electrons so that the outer shell electrons do not “feel” the entire positive charge of the nucleus.
Terms
- cationA positively charged ion, as opposed to an anion.
- ionAn atom or group of atoms bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution.
- anionA negatively charged ion, as opposed to a cation.
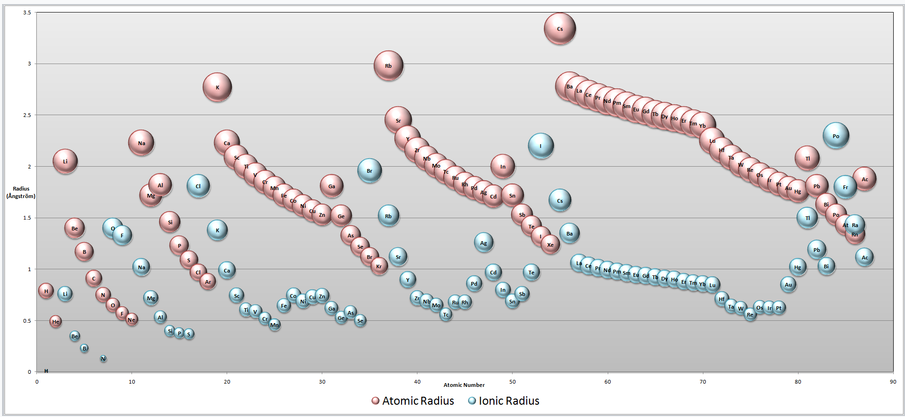
In chemistry, periodic trends are the tendencies of certain elemental characteristics to increase or decrease along a period (row) or group (column) of the periodic table of elements. Ionic radius (rion) is the radius of an ion, regardless of whether it is an anion or a cation. Although neither atoms nor ions have sharp boundaries, it is useful to treat them as if they are hard spheres with radii. In this way, the sum of ionic radii of a cation and an anion can give us the distance between the ions in a crystal lattice. Ionic radii are typically given in units of either picometers (pm) or Angstroms (Å), with 1 Å = 100 pm. Typical values range from 30 pm (0.3 Å) to over 200 pm (2 Å).
Trends in Ionic Radii

Ions may be larger or smaller than the neutral atom, depending on the ion’s charge. When an atom loses an electron to form a cation, the lost electron no longer contributes to shielding the other electrons from the charge of the nucleus; consequently, the other electrons are more strongly attracted to the nucleus, and the radius of the atom gets smaller. Similarly, when an electron is added to an atom, forming an anion, the added electron repels other electrons, resulting in an increase in the size of the atom.
The ionic radius is not a fixed property of a given ion; rather, it varies with coordination number, spin state, and other parameters. For our purposes, we are considering the ions to be as close to their ground state as possible. Nevertheless, ionic radius values are sufficiently transferable to allow periodic trends to be recognized.
As with other types of atomic radii, ionic radii increase upon descending a group and decrease going across a period. Note that this only applies if the elements are the same type of ion, either cations or anions. For example, while neutral lithium is larger than neutral fluorine, the lithium cation is much smaller than the fluorine anion, due to the lithium cation having a different highest energy shell.
Ionic Radii Of Transition Metals
Show SourcesBoundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
Ionic Radii Shannon
http://en.wiktionary.org/wiki/anion
Wiktionary
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/cation
Wiktionary
CC BY-SA 3.0.
http://en.wiktionary.org/wiki/ion
Wiktionary
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Ionic_radius
Wikipedia
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Periodic_trends
Wikipedia
CC BY-SA 3.0.
Sorted by Ionic Radius
Ionic Radii Of Transition Metals
| Name | Sym | # | |
|---|---|---|---|
| 0.012 Å | Hydrogen | H | 1 |
| 0.13 Å | Nitrogen | N | 7 |
| 0.23 Å | Boron | B | 5 |
| 0.35 Å | Beryllium | Be | 4 |
| 0.37 Å | Sulfur | S | 16 |
| 0.38 Å | Phosphorus | P | 15 |
| 0.4 Å | Silicon | Si | 14 |
| 0.46 Å | Manganese | Mn | 25 |
| 0.5 Å | Selenium | Se | 34 |
| 0.52 Å | Uranium | U | 92 |
| 0.52 Å | Chromium | Cr | 24 |
| 0.53 Å | Germanium | Ge | 32 |
| 0.535 Å | Aluminum | Al | 13 |
| 0.56 Å | Technetium | Tc | 43 |
| 0.56 Å | Rhenium | Re | 75 |
| 0.58 Å | Arsenic | As | 33 |
| 0.59 Å | Vanadium | V | 23 |
| 0.605 Å | Titanium | Ti | 22 |
| 0.62 Å | Gallium | Ga | 31 |
| 0.62 Å | Tungsten | W | 74 |
| 0.625 Å | Platinum | Pt | 78 |
| 0.625 Å | Iridium | Ir | 77 |
| 0.63 Å | Osmium | Os | 76 |
| 0.64 Å | Tantalum | Ta | 73 |
| 0.645 Å | Iron | Fe | 26 |
| 0.65 Å | Molybdenum | Mo | 42 |
| 0.68 Å | Ruthenium | Ru | 44 |
| 0.68 Å | Rhodium | Rh | 45 |
| 0.69 Å | Tin | Sn | 50 |
| 0.69 Å | Nickel | Ni | 28 |
| 0.69 Å | Niobium | Nb | 41 |
| 0.71 Å | Hafnium | Hf | 72 |
| 0.72 Å | Zirconium | Zr | 40 |
| 0.72 Å | Magnesium | Mg | 12 |
| 0.73 Å | Copper | Cu | 29 |
| 0.74 Å | Zinc | Zn | 30 |
| 0.745 Å | Cobalt | Co | 27 |
| 0.745 Å | Scandium | Sc | 21 |
| 0.75 Å | Neptunium | Np | 93 |
| 0.76 Å | Antimony | Sb | 51 |
| 0.76 Å | Lithium | Li | 3 |
| 0.78 Å | Protactinium | Pa | 91 |
| 0.8 Å | Indium | In | 49 |
| 0.848 Å | Lutetium | Lu | 71 |
| 0.85 Å | Gold | Au | 79 |
| 0.858 Å | Ytterbium | Yb | 70 |
| 0.86 Å | Palladium | Pd | 46 |
| 0.869 Å | Thulium | Tm | 69 |
| 0.881 Å | Erbium | Er | 68 |
| 0.887 Å | Plutonium | Pu | 94 |
| 0.9 Å | Yttrium | Y | 39 |
| 0.901 Å | Holmium | Ho | 67 |
| 0.912 Å | Dysprosium | Dy | 66 |
| 0.923 Å | Terbium | Tb | 65 |
| 0.925 Å | Einsteinium | Es | 99 |
| 0.934 Å | Californium | Cf | 98 |
| 0.938 Å | Gadolinium | Gd | 64 |
| 0.947 Å | Europium | Eu | 63 |
| 0.949 Å | Berkelium | Bk | 97 |
| 0.964 Å | Samarium | Sm | 62 |
| 0.97 Å | Tellurium | Te | 52 |
| 0.97 Å | Cadmium | Cd | 48 |
| 0.97 Å | Curium | Cm | 96 |
| 0.972 Å | Thorium | Th | 90 |
| 0.979 Å | Promethium | Pm | 61 |
| 0.982 Å | Americium | Am | 95 |
| 0.99 Å | Calcium | Ca | 20 |
| 0.995 Å | Neodymium | Nd | 60 |
| 1.013 Å | Praseodymium | Pr | 59 |
| 1.02 Å | Sodium | Na | 11 |
| 1.02 Å | Mercury | Hg | 80 |
| 1.03 Å | Bismuth | Bi | 83 |
| 1.034 Å | Cerium | Ce | 58 |
| 1.061 Å | Lanthanum | La | 57 |
| 1.1 Å | Nobelium | No | 102 |
| 1.119 Å | Actinium | Ac | 89 |
| 1.12 Å | Strontium | Sr | 38 |
| 1.19 Å | Lead | Pb | 82 |
| 1.26 Å | Silver | Ag | 47 |
| 1.33 Å | Fluorine | F | 9 |
| 1.35 Å | Barium | Ba | 56 |
| 1.38 Å | Potassium | K | 19 |
| 1.4 Å | Oxygen | O | 8 |
| 1.43 Å | Radium | Ra | 88 |
| 1.5 Å | Thallium | Tl | 81 |
| 1.52 Å | Rubidium | Rb | 37 |
| 1.67 Å | Cesium | Cs | 55 |
| 1.8 Å | Francium | Fr | 87 |
| 1.81 Å | Chlorine | Cl | 17 |
| 1.96 Å | Bromine | Br | 35 |
| 2.2 Å | Iodine | I | 53 |
| 2.3 Å | Polonium | Po | 84 |
[Last Updated: 2/22/2007]
Citing this page
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Periodic Table of Elements - Sorted by Ionic Radius. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/24/2021
https://EnvironmentalChemistry.com/yogi/periodic/ionicradius.html
.
Linking to this page
Ionic Radii Of Elements
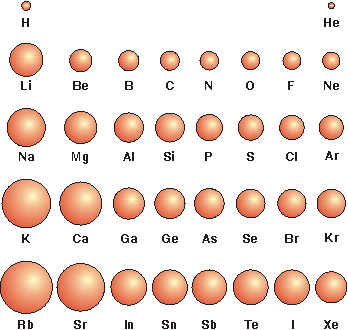
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/ionicradius.html'>echo Periodic Table of Elements: Sorted by Ionic Radius (EnvironmentalChemistry.com)</a>- This site offers comprehensive information for each element including: who, when & where; up to 40 properties (chemical & physical); over 3,600 nuclides (isotopes); over 4,400 nuclide decay modes; the element names in 10 different languages; and more. In addition chemistry and technical terms are linked to their definitions in the site's chemistry and environmental dictionary.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
Cl Ionic Radii
PLEASE, if you like an article we published simply link to it on our website do not republish it.




